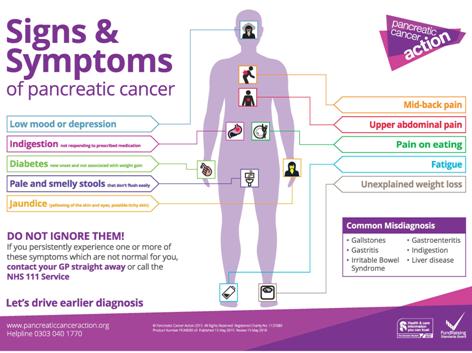
|
|
|
|
Topics List
Every topic is vital
& revealing reading
|
|
|
|
|
|
Minor
Breakthrough Potential
|
|
|
● Metastatic Stage 4: Some patients now eligible for
surgery
● Third-Line Therapy: Some Possibilities
● Chemo or Surgery First? New Thinking
● Add Cisplatiun to Nab-Paclitaxel+Gemcitabine?
● BRCA Treated By PARP Inhibitors
● PAXG (with Cisplatin) Performs Well
● How To Pick The Best Treatment Facilities
● Find The Best Clinical Trials
●
New NCCN
Guidelines & How To Use Them
●
mFOLFIRINOX Superior After Resection
●
Right To Try
Act: Experimental Drugs
●
US Healthcare
For Non-Citizens
|
|
|
|
|
|
Acinar
Cell Carcinoma (ACC) here
& here
|
|
|
|
|
|
Anaplastic,
Pleomorphic Carcinoma of the Pancreas
here
|
|
|
|
|
|
Adjuvant Chemotherapy (After Resection)
|
|
|
|
mFOLFIRINOX
– Labeled a “Breakthrough” at the 2018 ASCO Annual Meeting, here1,
here2
& here3
|
|
|
|
|
|
Anorexia & Cachexia (Prevent Wasting) here
|
|
|
|
|
|
Ascites here1, here2,
here3,
here4,
here5
& here6
|
|
|
|
|
|
ASCO Meetings & Symposia
|
|
|
|
2020 Annual Meeting,
June Abstract analysis soon
|
|
|
|
2020 Gastrointestinal,
January: Abstract
analysis
|
|
|
|
2019 Annual Meeting,
July: Abstract
analysis
|
|
|
|
2019 Gastrointestinal,
January: Abstract
analysis
|
|
|
|
2018 Annual Meeting,
June here
& abstracts
|
|
|
|
2018 Gastrointestinal,
January here
& abstracts
|
|
|
|
ASCO Clinical Practice
Guidelines
|
|
|
|
|
ASCO 2017 Stages
1 & 2 guide
|
|
|
|
|
ASCO 2016 Stage
3 guide
|
|
|
|
|
ASCO 2018 Stage
4 guide
|
|
|
|
|
|
Bacteria & Fungi Promote Pancreatic
Cancer
|
|
|
|
Breakthrough
research described
here
|
|
|
|
Bacteria
& Antibiotics: here1,
here2
, here3
& here4
|
|
|
|
|
|
Beta
Blockers Help here
|
|
|
|
|
|
Blood
Transfusion (harmful, avoid it), see
this
|
|
|
|
|
|
Care
Management
|
|
|
|
2018, available in the column to
the right
|
|
|
|
|
|
C.
diff Infection here
|
|
|
|
|
|
Chemotherapy Regimens
|
|
|
|
Best Adjuvant Therapy
(Post Resection) here
|
|
|
|
Best Maintenance Therapy (Post-Resection & Post
Adjuvant) here
|
|
|
|
Third Line Therapy here
|
|
|
|
Emerging Agents here
|
|
|
Other
Chemo Regimens follow:
|
|
|
|
New & Emerging
Regimens here
|
|
|
|
Which is the better
first therapy Nab-Paclitaxel + Gemcitabine or FOLFIRINOX? here
|
|
|
|
Which sequence is better,
NabP/Gem + NabP/Gem or NabP/Gem + FOLFIRINOX? here
|
|
|
|
FOLFIRI better than
FOLFIRINOX & FOLFOX? here
|
|
|
|
mFOLFIRINOX
better than FOLFIRINOX? here
|
|
|
|
|
Predict FOLFIRINOX
Response here
|
|
|
|
|
BRCA Role for FOLFIRINOX
& Cisplatin here
|
|
|
|
2018 ASCO Gastrointestinal
Symposium, Jan here
|
|
|
|
2018 ASCO Annual
Meeting, Jun here
& abstracts
|
|
|
|
Critical!:
Third-Line Therapy Regimens here
|
|
|
|
Add Cisplatin
to Nab-Paclitaxel/Gemcitabine here
|
|
|
|
|
Cisplatin: How to manage
harsh cytotoxics here
|
|
|
|
|
PAXG:
Good
performance was reported in 2018 for Metastatic Stage
4 & Locally Advanced Stage
3
|
|
|
|
|
Cisplatin requires hydration. Review
the topic: Wrong IV Bag here
|
|
|
|
BRCA Role for FOLFIRINOX
& Cisplatin here
|
|
|
|
Nab-Paclitaxel/Gemcitabine
Upgrades here
|
|
|
|
GABRINOX:
Merged Gem/NabP + FOLFIRINOX,
Abstract 4109 here
|
|
|
|
Chemo Regimens where
Nab-Paclitaxel is not available (as in some European nations) here
|
|
|
|
|
PEXG, PEFD & PDFG
Regimens here
& here
|
|
|
|
OXIRI & AGAP
Regimens here
|
|
|
|
Metronomic Chemotherapy
Schedule here
|
|
|
|
|
|
Chemo Agents, Emerging. This is NOT a priority list
|
|
|
|
Very Important: Study
the 2018 thru latest ASCO
symposia reviews which detail all emerging therapies
|
|
|
|
EGFR BATs performance is
detailed in the 2019
ASCO abstract analysis, and in the earlier Abstract 4108 here
and the 2015 report here
|
|
|
|
LOAd703 Tumor Injection: Good
interim results from this small soon-to-close clinical
trial
|
|
|
|
SM-88 is described in
the 2019
ASCO abstract analysis
|
|
|
|
AM0010 see
the bottom of this page
|
|
|
|
CPI-613 here
|
|
|
|
Napabucasin (BBI-608) here
& Phase 3 Trial here
|
|
|
|
TTFields (Tumor Treating
Fields) here
|
|
|
|
PEGPH20 details are given below
|
|
|
|
For Liver Metastases see
CAR-T injection therapy here; a
new clinical trial may be available in 2019
|
|
|
|
|
|
Clinical Trials
|
|
|
|
How to Select the Best, column at right
|
|
|
|
IRE + Chemo Trial at the
University of
Louisville (limited
to 20 patients), details here & here
|
|
|
|
Jan 2018 ASCO
Gastrointestinal Symposium details good ongoing trials starting here,
bottom of page
|
|
|
|
Jun 2018 ASCO Annual
Meeting describes good ongoing trials
starting here
|
|
|
|
Jun 2018 ESMO Meeting cites a few good trials here
|
|
|
|
|
|
ESMO European Society For Medical
Oncology
|
|
|
|
Jun 2018 Meeting here
|
|
|
|
|
|
Financial
Help
|
|
|
|
Travel & Support: Angel
Flight and CancerNet
|
|
|
|
Charity Grants: CancerCare
|
|
|
|
Drug Discounts: Contact the manufacturer’s customer assistance
team
|
|
|
|
|
|
Genetic
Testing here
& the discussion to the right
|
|
|
|
BRCA
Mutation
|
|
|
|
|
Role of FOLFIRINOX &
Cisplatin here
|
|
|
|
|
Early evidence of PARP benefit
is presented here
in 2014, here
& here
in 2016, here
in 2017 & here
in 2018
|
|
|
|
|
PARP-inhibitor Rucaparib
may help BRCA-defect patients, but appears to work better
as 2nd Line monotherapy
|
|
|
|
DDR Defects & PARP
Inhibitors, Abstract 4128 here
|
|
|
|
MSI, dMMR & Lynch
Syndrome, Abstract e16254 here
|
|
|
|
|
|
High Volume Facilities Are Life
& Death here
|
|
|
|
|
|
Hospital Threats
|
|
|
|
Wrong IV Solution here
|
|
|
|
Medical Devices Likely
To Hurt Patients here
|
|
|
|
Prevent
Hospital-Acquired Infections here
|
|
|
|
Prevent & Treat C.
diff Infection here
|
|
|
|
Immuno-Nutrition, taken before IRE or surgery here
& here
|
|
|
|
Prevent wasting (Anorexia
& Cachexia) here
|
|
|
|
|
|
Immunotherapy
(see also Targeted Therapy)
|
|
|
|
MSKCC’s Eileen O’Reilly
on Immunotherapy here
|
|
|
|
AM0010 here,
bottom of page
|
|
|
|
EGFR BATs Immunotherapy, Abstract 4108 here
& 2015 report here
|
|
|
|
Pamrevlumab (FG-3019), Abstract 4016 here
|
|
|
|
Pembrolizumab
(Keytruda) (PD-1 blocker): MSI, dMMR, Lynch
Syndrome, Abstract e16254 here
|
|
|
|
|
Beware:
In rare cases PD-1/PD-L1 blockers have caused rapid disease progression,
called hyperprogression, more here
|
|
|
|
|
|
Irreversible Electroporation
(NanoKnife®)
|
|
|
|
IRE
+ Chemo Trial at the University
of Louisville
(limited to 20 patients), details here & here
|
|
|
|
How IRE Works here
and see discussion below
|
|
|
|
Pancreatic Cancer &
Training Materials here
|
|
|
|
3D IRE Needle Guidance
System here
|
|
|
|
IRE vs Microwave
Ablation here
& here
|
|
|
|
Facilities
Offering IRE
|
|
|
|
|
Pancreatic Specialists
2016 here1
|
|
|
|
|
Manufacturer List here2,
here3
& here4
Disease
& Specialty (Surgeon Vs Radiologist) are revealed in List-1, but not in
Lists 2-4.
|
|
|
|
|
Some IRE Practitioners
in Europe here
|
|
|
|
Nanoknife Surgery
Warriors support group
& below
|
|
|
|
|
|
Liver
Metastases
|
|
|
|
Infusion Chemotherapy here
|
|
|
|
IRE Irreversible
Electroporation here,
here
& here
(near bottom of page)
|
|
|
|
|
|
Lung Metastases Removed here
& here
|
|
|
|
Manageable if limited to
lung here,
here
& here
|
|
|
|
Ablation Methods here,
here
& here
|
|
|
|
|
|
Lymph Node Metastases Removed here, here
|
|
|
|
|
|
Metastases Removal If Few Sites here
& here
|
|
|
|
|
|
Microsatellite Instability (MSI), Mismatch
Repair Deficiency (dMMR) & Lynch Syndrome
|
|
|
|
Pembrolizumab
(Keytruda): Anti-PD1/PDL1 Checkpoint Inhibitor, Abstract e16254 here
& at right
|
|
|
|
|
Read about disease
hyperprogression above
|
|
|
|
|
|
NCCN Clinical Practice Guidelines, Use
Them
|
|
|
|
NCCN 3.2019 is downloadable
here
|
|
|
|
ASCO Clinical Practice
Guidelines
|
|
|
|
|
ASCO 2017 Stages
1 & 2 guide
|
|
|
|
ASCO 2016 Stage
3 guide
|
|
|
|
ASCO 2018 Stage
4 guide
|
|
|
|
|
Pain
Management: An international group offers a comprehensive
guide
|
|
|
|
|
|
PanCAN’s Know
Your Tumor Program here
|
|
|
|
PanCAN’s Know Your Tumor program fails to
improve the chief measure of success: Overall Survival. Read the Conclusions in this latest trial report. And, read more about Targeted Therapy here.
|
|
|
|
|
|
Pancreatic
NET’s (NeuroEndocrine Tumors) here
|
|
|
|
|
|
PEGPH20 (Trials of Metastatic Patients)
|
|
|
|
Fails when used with
FOLFIRINOX here
|
|
|
|
Helpful when used with
Nab-Paclitaxel + Gemcitabine, but only in HA-High patients here
|
|
|
|
|
|
Peritoneal
Metastases are Treatable
|
|
|
|
Study Abstract
702 and the posting here
|
|
|
|
Study Abstract P-137 here
|
|
|
|
|
|
PERT dramatically extends
survival time
|
|
|
|
Pancreatic patients
often suffer from Pancreatic Exocrine Insufficiency (PEI). Fortunately, new
2018 research finds that survival time can be extended 50 to 100% by the
treatment of PEI
using Pancreatic Enzyme Replacement Therapy (PERT).
|
|
|
|
|
|
Precision
Medicine (Targeted Therapy) here
|
|
|
|
|
|
Proton Therapy (Particle Radiation) here
|
|
|
|
Advantages here
|
|
|
|
Proton Therapy Centers,
list here
|
|
|
|
Lung treatment here
|
|
|
|
Lymph Node treatment here
|
|
|
|
Temporarily, UK’s NHS funds some Proton Therapy in the USA
plus travel, see this,
this
& this
|
|
|
|
|
|
Radiation (This is X-Ray Photon, not Proton)
|
|
|
|
Case For: Available here soon
|
|
|
|
Radiation does NOT
benefit metastatic patients, see Abstract
e16257 here. And it does NOT benefit R1 resection
patients, the report is here
& review here
|
|
|
|
Case Against here
|
|
|
|
ChemoRadiation here
|
|
|
|
2018 Gastrointestinal
Symposium here
|
|
|
|
Warning: Fiducial
insertion could seed malignant cells unless a peripheral path is used, see this
|
|
|
|
|
|
Resection
/ Surgery
|
|
|
|
Surgery Scope &
Details
|
|
|
|
|
Surgical Resection
options
|
|
|
|
|
Prevent
Complications
|
|
|
|
|
Minimally-Invasive
Techniques
|
|
|
|
Surgery-First vs
Neoadjuvant-First here
& here
|
|
|
|
New Maintenance Therapy
After-Surgery here
|
|
|
|
Pasireotide halves
resection-related risk here
|
|
|
|
ImmunoNutrition before surgery here
& here
|
|
|
|
|
|
Right
To Try Act here
|
|
|
|
|
|
Sleep
Method
|
|
|
|
Bright Light Sleep
Therapy here
|
|
|
|
PhilipJax Sleep Method here
|
|
|
|
|
|
Stents
to Manage Biliary & Duct Obstruction
|
|
|
|
Ensure that stents are
removable, to allow IRE
later
|
|
|
|
|
|
Surgery-First vs Neoadjuvant-First here
& here
|
|
|
|
|
|
Symptom
Management here
|
|
|
|
Control Venous ThromboEmbolis (VTE) here
|
|
|
|
|
|
Targeted Therapy (Precision Medicine)
|
|
|
|
Current status of Targeted Therapy detailed here.
|
|
|
|
|
This 2018 NY Times story
describes the uncertainty of Targeted Therapy.
|
|
|
|
|
PanCAN’s Know Your Tumor program fails to
improve the chief measure of success: Overall Survival. Read the Conclusions in this latest trial report. More on the PanCAN project here.
|
|
|
|
Some active Targeted
Therapies
|
|
|
|
|
MSI, dMMR & Lynch
Syndrome Immunotherapy, Abstract
e16254 here
|
|
|
|
|
EGFR BATs Immunotherapy, Abstract 4108 here
& 2015 report here
|
|
|
|
|
|
Third-Line Therapy
|
|
|
|
LV5FU2 + Nab-Paclitaxel & Others here
& here
|
|
|
|
Erlotinib + Gemcitabine
here1,
here2,
here3
& the sequence algorithm here4
|
|
|
|
A
possibility for some patients: Nanoliposomal Irinotecan (MM-398) + 5FU (or
Capecitabine) here
|
|
|
|
PARP-inhibitor
Rucaparib
may help BRCA-defect patients, but works better
as 2nd Line monotherapy
|
|
|
|
NabP+G is a possible 3rd line
therapy even after 1st line NabP+G, see Abstract P-147 here
|
|
|
|
3rd
Line regimens may be gleaned from this
2014 article. The 3rd Line search vexes
oncology leaders
|
|
|
|
|
|
Transfusion (harmful, to be avoided), see this
|
|
|
|
|
|
Vitamin D (Paricalcitol, aka Zemplar®) here
|
|
|
|
|
|
|
|
|
Right
To Try Act
|
|
|
One Hundred Fifteenth Congress
|
|
|
Click the following to view
|
|
|
|
●
|
The Right To
Try Act
|
|
|
|
●
|
Excellent Legal
Background Paper
|
|
|
|
●
|
CSPAN Interviews Chief RTT Advocate
|
|
|
On May 22, 2018 Congress passed (and
on May 30 the President signed) legislation which allows an “eligible patient” (one suffering
from a “life-threatening
disease or condition”) to access “unapproved
medical products” (also called an “eligible
investigational drug”) without FDA approval.
The Right To Try Act (RTT
Act) makes it easier for people to access experimental treatments outside
of a clinical trial. Not all the
details are yet known. However, the
following is certain:
1.
The patient
must have “exhausted approved
treatment options” “as certified
by a physician” and must be “unable
to participate in a clinical trial involving the eligible investigational
drug.”
2.
The “eligible investigational drug” must
be the focus of a completed Phase 1 (or later) clinical trial.
3.
It is not
certain what TYPE of medical intervention constitutes an ELIGIBLE
investigational “drug.”
It is not known for example whether
Stem Cell infusion, Irreversible Electroporation, Proton Therapy or TT Fields might be considered a “drug.” The answer will be found in existing
Congressional acts, since the drug must be “the subject of an active investigational new drug application
under section 505(i) of this Act or section 351(a)(3) of the Public Health Service Act.”
However, some of the promising Emerging Agents, listed
above, will likely be available outside clinical trials.
Updates are given below.
|
|
|
|
|
|
RTT
update. The Right
To Try Act does NOT mandate
manufacturer cooperation. The
Act states:
“It is the sense of the Senate that . . . [the Act] does not establish any
new mandates, directives, or additional regulations.”
Neither FDA’s current “Expanded
Access” (Compassionate
Use) program nor the new Right
To Try Act compels participation by drug companies. However, the new Act does offer some
slight advantages:
●
Right To Try bypasses FDA’s approval process for Compassionate
Use of experimental drugs.
Patients now need only the approval
of their physician and the
drug manufacturer. Time and red tape are saved.
●
The new law protects doctors and companies from the legal risks of allowing
unapproved treatments, unless they intentionally harm a patient.
Critics are correct that, in many
respects, the Act is dangerous:
●
Many Phase 1
drugs (the likely choice by patients under this Act) do not succeed in
later clinical trial phases – some are later found to be harmful.
●
In addition,
when a patient selects a treatment under this Act, he could easily forsake
a better therapy, one which has benefits proven by more thorough
research. So time and opportunity
are lost – meanwhile the disease progresses.
●
Further, the
patient will likely have to pay for the new drug, which may not be covered
by insurance, often impoverishing the patient and achieving nothing.
●
And, of
course, some therapies cannot be mass-produced, so may be unavailable at
any price.
The above assessment is early. Time may reveal better features of this
law. But, it is certain that drug
manufacturers are NOT required to participate.
A better law would allow patients to
use drugs which are FDA-approved for other applications. For example, some drugs, approved for
lung cancer or ovarian cancer, are not FDA-approved for pancreatic
cancer. Yet some emerging pancreatic
chemo regimes might employ those lung/ovarian drugs. However, their costs are not covered by
insurance because they lack FDA approval for the pancreatic application.
|
|
|
|
|
|
|
|
|
Keywords: best-pancreatic, new-pancreatic,
pancreatic-cancer-cure, pancreatic-cancer-development,
best-drugs-for-pancreatic, targeted-therapy, precision-medicine,
best-clinical-trials, care-management, irreversible-electroporation,
lung-metastases, nccn-guidelines, third-line-therapy,
chemotherapy-regimens, cachexia, anorexia, philipjax, folfirinox,
nab-paclitaxel, hospital-acquired-infection, ascites, symposium,
chemo-agents, proton, pembrolizumab, egfr, microsatellite-instability, dmmr, hyperprogression,
right-to-try.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rules for Patients &
Care Managers
|
|
|
|
“A Lack Of
Wisdom Is Fatal”
|
|
Care Management. Below are
practices that should be adopted when dealing with a life-taking disease.
1.
Family Researcher. The
family must identify at least one bright, skilled person, with excellent
computer savvy, to devote 100% of his time
to care management – and the family must support that person by taking over
the normal work and home responsibilities which he now must abandon. The lack of such good organization costs
lives.
2.
Care Manager. The family Care Manager
must maintain updates on all reports at
all times: Overall condition, surgery, biopsy, PET/MRI/CT,
bloodwork, etc. And, be prepared to
convert them immediately into one clean, logically-sequenced pdf file. Software can help the manager – Fax, pdf
and definition software.
Fax. For a
hospitalized patient, often the surest way to reach a physician is via
Fax. A physician will always read a
fax. All medical offices and nursing
stations are Fax-equipped. There are
online services, like eFax, and PC-based software like FaxTalk and (for
WinXP) WinFaxPro. Win8 and Win10
have built-in software, but you will need a $10 USB modem (buy on eBay) and
a landline.
PDF
conversion. PDFCreator will convert most documents
(graphic or text) to pdf, available free here.
Definitions. Many
medical (and other) terms are defined for you via free WordWeb, just Ctrl Right-Click over a difficult word and get
the definition. Free here.
3.
Planning Is Critical. The Care
Manager and patient must chart a COMPLETE course from the very beginning.
For example, make sure that all metal
stents are removable so that they can be extracted before an IRE procedure. Metal
will distort IRE’s electrical field.
Further, there must always be
multiple irons in the fire, so the patient can jump immediately to another
best therapy when one BEGINS to fail.
Too often managers make the fatal
mistake of waiting until one therapy fails before starting the
search for another.
An “iron” is in the fire ONLY if the
therapy is suitable; the patient is eligible, and the related physician is in
agreement. “Thinking about it”
doesn’t count as an iron. See Item
7, below, for therapy sequencing.
4.
Discard Managed Health Plans. If the
patient has a managed plan, be certain that the major out-of-state cancer
centers (those highest
ranked by US News) are available (which is not likely). If an unlimited-access
plan can be had, get it.
If Medicare can be changed BEFORE diagnosis, do so. Avoid Medicare Advantage, since it often
prohibits access to the highest-ranked cancer centers. Get
Original Medicare plus 100% Medigap plan F or G. The Medigap puzzle is addressed
here.
●
From Link-1
it appears that you can switch from Advantage to Original Medicare during
the period January 01 through February 14 each year or PERHAPS October 15
through December 07. But Medigap
complicates the issue. Keep reading.
●
And,
according to Link-2,
you can be granted a Special Enrollment
Period (SEP), if you enrolled in the Medicare Advantage plan based
upon misleading or incorrect information provided by plan materials,
employees or insurance agents. Link-2
covers Ohio,
but the conditions are the same in all 50 states.
●
It is
possible that you don’t actually have to PROVE that you were misled. Your reasonable, unwavering instance may
be enough. Press hard; you have
nothing to lose.
Further, Medicare patients need a Medigap plan that pays the 20% which
Medicare does not cover. Medigap
Plans F & G do that. Plan G does
not cover the $183 Part B deductable, but its price savings nearly always
exceeds the $183 deductible, so offers a savings opportunity.
Finally, switching to Medigap AFTER diagnosis is
complicated. Many of the issues are addressed
here.
It is possible to purchase Medigap
BEFORE leaving managed Medicare, giving the buyer a chance to learn whether
he will be issued Medigap.
If you can achieve it, Original Medicare plus Medigap will assure that you
are welcomed financially at the best care
facilities.
5.
US Healthcare For
Non-Citizens. See the section below: US
Healthcare For Non-Citizens.
6.
Financial Help. Travel and Financial Aid may
be available through Angel
Flight and CancerNet. Charity grants can be found through CancerCare. And drug discounts and donations often
can be had by contacting the manufacturer’s customer assistance team.
7.
Best Facilities. The
general public tends to believe that all physicians have equal competence
and equal skills, and thus they select a local surgeon to manage
life-threatening illnesses. A
mistake: Community hospitals should be used for broken bones and stitches,
not for the treatment of life-threatening diseases. For deadly cancers, treatment should be
sought at major cancer centers, those highest
ranked
by US News – the only exception being IRE facilities. Not every institution that calls itself a
“cancer center” is indeed one.
Resectability (the only chance for
cure) is often determined by the skill of the surgeon. MD Anderson Cancer Center explains here. Read about the advantages of “high volume” hospitals here.
Certainly, travel is inconvenient,
but death is far more inconvenient.
We must seek help from those who
write the books, not from those who won’t find the time to read
them.
For example, only the most skilled
and experienced IRE practitioners should be used. IRE has a significant learning curve. If the placement of probe-pairs is
incorrect or the pair spacing is 5mm outside specifications, IRE ablation
will be imperfect, as noted in this
report.
8.
NCCN Guidelines. Study (not read) the NCCN
Guidelines in the sequence recommended here. In one day’s hard work the family Care
Manager will be well-acquainted with therapy, and via the Authors and
References will learn the names of oncology leaders to consult. Reprehensibly, more than half of all oncologists
fail to follow the guidelines.
9.
Therapy Sequence. Sequence
and timing are critical. It is vital
that the patient or Care Manager (not the treating physician) selects the
best therapy path right from the beginning
(with adjustments along the journey).
See Many
Irons in the Fire, below.
This is a swiftly moving parade – one misstep and you cannot go back
and take a path previously forsaken.
For example:
9.1.
Most (but not
all) clinical trials require patients to be therapy naïve (that is, no
prior therapy). So, at the moment of
diagnosis, the Care Manager (skilled friend or relative) must begin an
intensive self-education and search effort.
That effort
requires a solid week of hard work.
But, that’s how this website helps.
See the section below on prudent Clinical
Trials. There is no time for
hand-ringing.
And, the
physician will NOT do this work for you.
In most community settings his path
is predicable: Start with First Line Therapy; when that fails employ
a Second Line Therapy, and when he runs
out of ideas send the patient to his radiation colleague to share
the wealth.
9.1.
Further, for initially-resectable patients recent
research (presented at the 2018
ASCO meeting) argues that mFOLFIRINOX is the best adjuvant (post
surgery) therapy. That means that
Nab-Paclitaxel + Gemcitabine should be considered for initial neoadjuvant
(pre-surgery) chemotherapy. Neoadjuvant therapy is
now recommended, rather than immediate surgery for those patients
who are resectable at diagnosis.
Unfortunately, most patients are metastatic (not resectable) when
first diagnosed.
9.2.
If possible, Irreversible Electroporation (IRE) should be undertaken as
part of open surgery and before any radiotherapy. Leading IRE surgeon Robert CG Martin, MD,
PhD cautions in this 2016
journal article: “IRE . . . will
not be successful as a salvage therapy . . . patients who have already gone
through extensive surgical dissection are not suitable candidates [and] IRE
should be used with caution after high dose radiation therapy because of
the significant damage that the radiation therapy induces . . . ”
IRE might also
be used for lymph nodes and for liver
metastases if there are 3 or fewer lesions in one organ. Lung metastases can
be managed, but not by IRE.
10. Many Irons In The Fire. Most
families tend to seek care in SERIES, meaning that they seek treatment at
one location, and, when it fails, BEGIN the search for the next path – with
an enormous loss of life-taking time.
This is a great mistake. One must have multiple irons in the fire (called “contingency planning”),
by which the family has prepared MULTIPLE institutions to undertake the
next chapter of treatment.
●
Getting input from distant physicians, without traveling, is
possible, if you are organized and have computer skills (and have been wise
enough to collect the key diagnostic and treatment reports).
Gather the latest key reports. Convert them into pdf format. Make them easy to read, large and
oriented properly – and remove all static and blemishes from the pages. Send them by email to IRE practitioners
in ONE multi-page file, in logical order: Overall condition report first,
then surgery, then MRI/PET/CT, then biopsy, bloodwork, etc. Use a cover letter which gets to the
point in about 6 sentences and avoids sentimentality. Don’t waste the physician’s time. For example:
Dear
Dr X, Will you consider accepting X under your care? He suffers from X and has been treated
with X. He is physically strong and
well-insured. Attached are the
current reports. I am indebted to
you for your help.
●
When scanning
documents, set the TWAIN preferences to “line art” or “line drawing”, not
“color”, to produce a cleaner B/W product; then save it as .pdf, .png,
.bmp, .tif or .gif file, but not .jpg, since a .jpg will smear the pixels.
11. Research. There are
four principles to practice:
●
The patient
and family must be so well educated
about therapy that they know in advance
of the next physician meeting what the physician will recommend.
●
You must
fight hard to get any new therapy.
If you don’t seek it, you won’t get it.
●
Many family “Care Managers” think that they will find a
physician who will do all the searching and future arrangements for them –
those managers are often lazy and looking for an excuse to avoid hard
work. There is no such magic
physician. And, if you find one, you
wouldn’t know whether he is correct unless you yourself do the
research. A life is at stake; trust
no one else.
●
If the physician disagrees with the medical literature (NCCN and journal articles), the physician is
wrong. In medicine Truth and Falsity
are determined by carefully-designed clinical trials. No amount of physician anecdotal
experience can override the findings of clinical trials.
12.
Genetic Testing. At the
time of diagnosis genetic testing should be undertaken. So urges Cedars-Sinai
Medical Center
(Abstract 4128) and Stanford
University Medical Center (Abstract e16254) via 2018
ASCO Meeting reports.
Some genetic defects are now somewhat
druggable, including BRCA, DDR and especially Microsatellite Instability (MSI), Mismatch Repair
Deficiency (dMMR) and perhaps Lynch Syndrome. Find details in Abstracts 4128 and e16254
explained in the commentary here. The tests are identified in these
documents: MSI
and BRCA.
13. IRE
Facilities. This
2016 list identifies practitioners
targeting pancreatic tumors.
And, the following lists from
manufacturer AngioDynamics identify IRE facilities. But, they do NOT necessarily name those
facilities which target pancreatic tumors, nor do they specify whether the
practitioner is a surgeon or an interventional radiologist. AngioDynamics: 2016
document, its 2016
spreadsheet and its online
facility search.
14. Hospital
Admission. Accept financial responsibility for an
incapacitated patient ONLY if he is a bona fide legal dependant.
Never, under any
circumstances, sign for a non-dependent
(including parents) during hospital admission. This is how the facility hopes to bind
you to patient costs. If the patient
is incapable of signing, just write “Mr
XXX is unable to sign” in the signature space, and don’t draw attention to it. Every US hospital is obliged to treat
the patient without your legal commitment.
15. Hospital-Acquired
Infections. Infection can cause life-taking delays in
cancer therapy. Read about IV
solutions, C. diff and hospital acquired infections here.
The family must make sure that prevention measures are followed. And, if an infection occurs, the family
must understand the remedy thoroughly to assure that the treatment is
correct. Medical mistakes are too
common.
|
|
|
|
|
|
|
|
US
Healthcare For Non-Citizens
|
|
Obamacare MIGHT be a
vehicle for non-citizen access to US healthcare. And, it might be acquired in a few
months.
The US ACA
Affordable Care Act (ObamaCare) claims to ignore preexisting conditions, so
existing pancreatic cancer should be covered.
Further, ACA
is available to aliens who possess “nonimmigrant visas” and who are “lawfully present” in the US.
Recent federal rules may have reduced
the number of geographic areas where ACA is
reasonably-priced or active. Nevertheless,
this is a strategy that should be considered earnestly.
Details on how to qualify are available here.
Applications for nonimmigrant visas can be made online at this
site and this site. And, frequently asked questions are answered
here.
In addition, this
non-government site offers some insights which may be true, but which should
be verified at the government sites.
ObamaCare’s open enrollment period
begins in November. However, there may be exceptions,
allowing Special Enrollment at other times.
Learn more about enrollment periods at these three sites: At this site,
at this site
and this site
With some online work a non-citizen
might be treated in the US
before long.
It would be prudent to pursue the
visa and Obamacare plan, even if you are uncertain whether you will use
them. This is part of the “contingency planning” and the “irons in
the fire” which every pancreatic patient must practice at all times.
Before selecting a specific insurance plan, make
sure your preferred US
medical institutions will accept it.
|
|
|
|
|
|
How to Find the Best Clinical Trials. We are
always urged to seek clinical trials.
However, there must be great caution with this particular
disease. As noted oncologist Philip
A. Philip
remarked recently: “Clinical
trials in pancreatic cancer have been famous for being negative. [After] many clinical . . . trials, with
thousands of patients and millions of dollars in costs . . . I would count
maybe five clinical trials [in 20 years] . . . that led to . . . adoption
in our daily practices.”
During those 20
years nearly all trials led to nothing.
And, every failed human trial began as successful non-human research.
So, how should we evaluate clinical
trials?
First, look for agents which ADD TO
therapies already known to be effective.
Then, participate in the trail ONLY if the new agent has shown value
in earlier trial phases. There are
usually 3 trial phases. Dosage and
efficacy are usually determined in Phases 1 and 2. So, at the end of Phase 1 or 2, some
effectiveness will be known.
Thus, we want to find the agents and
practices which will LIKELY produce the greatest impact.
Download the excellent journal
article, titled: Pancreatic cancer:
Optimizing treatment options, new, and emerging
targeted therapies, 2015
Chiorean
& Coveler.
Its tables give comparative data on
current and promising agents. The
data provide only a rough comparison.
And, of course, a seemingly good regimen may not be suitable, if the
patient’s disease has progressed on the regimen’s principal component (like
Gemcitabine or Irinotecan).
Then, search the Clinical Trials
website here.
1.
Select “Open
Studies”
2.
Then, enter
key search terms. Place “pancreatic”
on the Conditions line, without
quotations marks.
3.
Enter standard therapies
(one at a time) on the Interventions line.
You could enter Gemcitabine, Irinotecan, FOLFIRINOX (FOLFIRINOX will
also find FOLFIRINOX alternatives), etc.
This way you are more likely to find new therapies combined with
acceptable mainstay therapies (also called backbone therapies).
4.
If you have
undergone resection or IRE, you might also put “resected” on the Search
Terms line. This approach does not
guarantee a perfect search of trials, but it is a good start.
5.
The search
will produce a column of results. If
you see a trial of interest, right-click and open a new tab. That way, when you close a tab, you
preserve the original search response page.
If you don’t use a mouse, get one. It will reduce errors and save time.
6.
When
reviewing the various trials look for trials that have a known, reliable backbone
regimen (FOLFIRINOX or Nab-Paclitaxel+Gemcitabine) with and
without the experimental agent. That
way the patient gets some good therapy, even if the new agent does nothing;
7.
If data on
the experimental agent is missing from the Chiorean-Coveler
article, look for trials in Phase-2 or later, that way there may be
results in Phase-1 to review. This
is usually the best approach, although there can be exceptions. Also, Phase-3 trials are usually randomized,
meaning that about half the patients do NOT get the experimental therapy
(at least not initially).
8.
Look for
large multi-institutional trials, which often identify a “breakthrough”
drug (or, on the negative side, they may simply identify a wealthy
manufacturer).
9.
Read the Inclusion and Exclusion criteria carefully; when in doubt contact the
researcher (an email address is usually given), using the most concise
email possible – get to the point in two sentences or less; for example: “For this trial will you accept a
locally-advanced patient who has recently undergone resection and who has
good performance status?”
10.When you find an experimental therapy of interest
to you, search Google (or PubMed), placing the name of the new therapy and
the word “pancreatic” on the Google word line.
Then, in the column of search results,
if you see a response of interest, right-click and open a new tab. That way you preserve the original search response page, and you can
open new tabs while the other tabs are loading. A mouse is essential for this search.
Any other approach is unwise. There are no cures at present. Therapy
agents should be chosen BY THE NUMBERS. If the performance
numbers: Response Rate (RR), Progression Free Survival (PFS), and
Overall Survival OS) are not known, you take great risk, forsake an
opportunity to use a better therapy,
and lose time which can never be recovered.
Meanwhile, the disease progresses.
To be prudent do not rely on
preclinical (non-human) studies.
Every failed human pancreatic trial began with a successful
preclinical study. In the past 20
years hundreds of human trials have failed, the vast majority of them. And the very few successful trials
produced only four chemo regimens now used in clinical practice – four
regimens in 20 years.
Occasionally you will discover a
trial which tests a new agent on nearly all cancers: Breast, colon, lung,
kidney, pancreas, head and neck.
Beware. The manufacturer
doesn’t know what organ will benefit if any. So, he wants to find out cheaply by
callously squandering the lives of you and other human guinea pigs. Exceptions to this warning are (1)
experimental agents applied to a specific genetic defect like BRCA or (2)
agents applied to a class of organs, such as the gastrointestinal system
(pancreas, liver, intestine, colon).
Do not choose a trial just because it
is offered or is local. Find the BEST one available on earth, and travel
to it, if necessary. Far more
inconvenient than travel is death.
If you seek a trial recommendation
from your medical oncologist, ask him to identify the BEST one available regardless of geographic
location. If he names a trial
that he administers, consider: What a monumental coincidence that the BEST
of all clinical trials on earth is being conducted by your oncologist (one
among the multitude of oncologists), who is also being paid to find trial
subjects. That physician may not be
your friend, no matter what he pretends to be.
Often, oncologists
make a “God’s judgment”: Shall I make every effort to treat this
patient responsible and cleverly, which might help her somewhat but
postpone the inevitable, or should I use her life in an uncertain trial
which may help all mankind?
Frequently, they push the patient
into the trial without telling her the grave uncertainties. And, sometimes these oncologists prod
patients into trials which they administer (trials which are not the BEST
available) – that conflict-of-interest is unethical and frequent.
|
|
|
|
|
|
Other Guidance.
1.
If the NUMBERS for a specific therapy don’t exist, you are squandering an opportunity offered by a
different, NUMBERS-rich therapy.
Wise financial investors review the past performance of stocks and
funds before investing. But, when it
comes to a life and death decisions, they cast away that wisdom. The numbers are Response Rate (RR),
Progression Free Survival (PFS) and Overall Survival (OS).
2.
Do NOT base your therapy decision on preclinical (non-human) trials.
Every failed human trial, of which there have been hundreds in the
past 20 years, began with a successful non-human study. And the very few successful clinical
trials produced only four chemo regimens now used in clinical practice –
four regimens in 20 years. The failed
human trials cost many millions and enrolled thousands of patients – most
of whom sacrificed themselves and gained
nothing. Select the therapy wisely,
based on the NUMBERS.
3.
Do not be
seduced by a researcher’s use of the term “significant”, as in “significant increase” or “significant improvement.” He is often referring to statistical
significance, a technical term in probability theory. For example, an experimental agent might
add 1 week to Overall Survival, the 1-week being “statistically
significant”, but not a significantly long time in our quest.
4.
Urge PET scans, early and
frequently. In February 2018 NICE,
the guidance agency for the UK
socialized health care system, began
recommending early PET scans.
NICE is the misnamed National
Institute for Clinical Excellence.
|
|
|
|
|
|

|
|
Nanoknife Surgery
Warriors. The Warriors Facebook forum
was started several years ago by David Shell who recognized the need for an
IRE information exchange – to educate and to bolster past and future Irreversible Electroporation (IRE)
patients.
It is an excellent support group;
but, unlike this site, it is not a reliable source of medical information.
With the help of IRE Mr Shell
survived four years against this terrible disease. He passed away Feb 20, 2018.
In 2016 he told his story here,
of ineptitude and confusion at major cancer centers. Incidentally, it is a false claim by
others in the story that IRE performs better following radiation.
|
|
|
|
|
|
Irreversible Electroporation
(IRE), a type of knifeless surgery,
is the most significant therapy advance in
20 years, allowing many Stage 3 Locally-Advanced patients to become
resectable, and improving surgical margins.
IRE became commercially available for research purposes in
2009. Robert
C.G. Martin, MD, PhD, FACS, Director of Surgical Oncology,
University of Louisville Medical School, is the principal developer of IRE
techniques for pancreatic cancer.
IRE, in the form of Nanoknife®, is a
soft tissue ablation method using ultra short but strong electrical fields
to create permanent, lethal nanopores in the cell membrane. The
advantage of IRE lies in its ability to ablate unwanted tissue
without harming vital protein-sheathed structures such as blood vessels,
ducts and nerves. This
tissue-selectivity, as well as real-time monitoring capability and sharp
ablation margins are its superior features.
In the region beyond its ablation
zone the cell poration is reversible, allowing chemo agents to enter more
easily before the pores eventually close.
IRE is performed by an interventional
radiologist percutaneously (through the skin) or by a surgeon
laparoscopically or intraoperatively (as part of open surgery).
The surgical
approach allows greater precision, which is paramount – the ablation
needles must be positioned
within 5mm of required spacing, or the ablation will be imperfect. To achieve that precision proficient IRE
practitioners employ the new 3D
needle guidance system.
An excellent introductory paper on
IRE is available for download here,
and other IRE information is here. Pancreatic IRE may be covered by Medicare
and private insurance under CPT
Code 49203.
Metal stents must be extracted, so that IRE’s electrical current
is not misdirected by the metal. So,
use removable stents; their manufacturers
are identified here.
IRE is being evaluated for the
treatment of bone and brain tumors, as well as other soft tissue tumors.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|